Top 6 Medical AI Models that You Need to Know
A.I & Engineering
Lately, it feels like everyone’s buzzing about the latest generative AI models like Stable Diffusion and large language models (LLMs). The hype is real, and the general public is captivated. But hiding under the radar of most people is the advancement of AI in the medical field.
The research and application of AI in the medical field has been breaking ground for over a decade, quietly revolutionizing healthcare behind the scenes. While the spotlight often shines on these flashy, new technologies, the unsung heroes of medical AI have been making strides that save lives and improve patient care every day.
AI in healthcare isn’t new; it’s a rather well studied and researched area. In fact, one of the biggest data science and machine learning platforms, Kaggle, has been hosting frequent medical competitions for the past decade, successfully engaging a global community to solve complex medical problems.
From curing an 82-year old of blood cancer that humans could not intervene using drugs discovered by AI to thousands of people receiving personalized "cancer vaccines", there are plentiful success stories.
AI models are now capable of interpreting medical images with accuracy that rivals human experts, predicting patient outcomes with unprecedented precision, and even aiding in the discovery of new drugs. These advancements are not just theoretical; they are making tangible differences in patient care and outcomes. Despite this, the buzz around generative AI often overshadows these critical developments.
With that being said, here are the top 6 advancements that you might have missed in the medical AI field.
The Beginning: AlphaFold
AlphaFold is perhaps one of the most well-known AI-related advancements to experts in the field, yet rather unknown to the general public. It’s one of the first major success milestones for AI in the medical field.
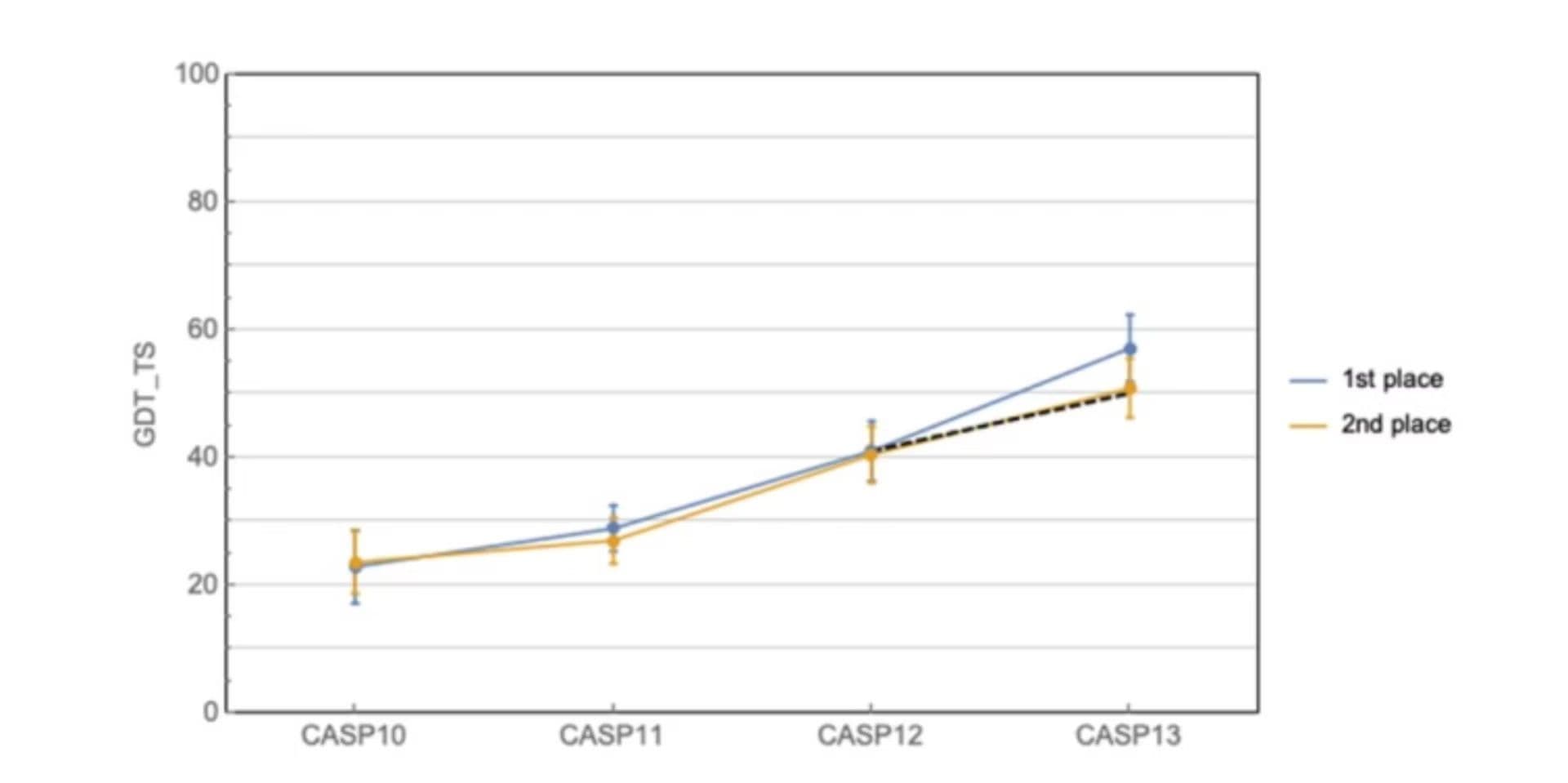
AlphaFold’s story started back in 2018 by placing first in the overall rankings of the 13th Critical Assessment of Structure Prediction (CASP). The CASP is a world-wide experimental competition for automated protein structure prediction that has taken place every 2 years since 1994. Many view the honor of winning the competition as taking the “world champion” in that field of science. When AlphaFold took first place by a historically large margin, it definitely shook the scientific community.
Graph showing best and second best predictions at CASP with the dashed line indicating the projected improvement to be observed at CASP 13.
Before we talk about how AlphaFold works, let’s first dive into why protein structure prediction matters so much.
Proteins are like tiny machines in our body that do a lot of important jobs other than being “healthy”, from building muscles, fighting off infections to digesting food.
However, for proteins to do their job, they need to be in the correct shape or structure. Think of it like a key fitting into a lock. If the key (protein) isn’t shaped correctly, it won’t work. This process of a protein taking its correct shape is called “protein folding.”
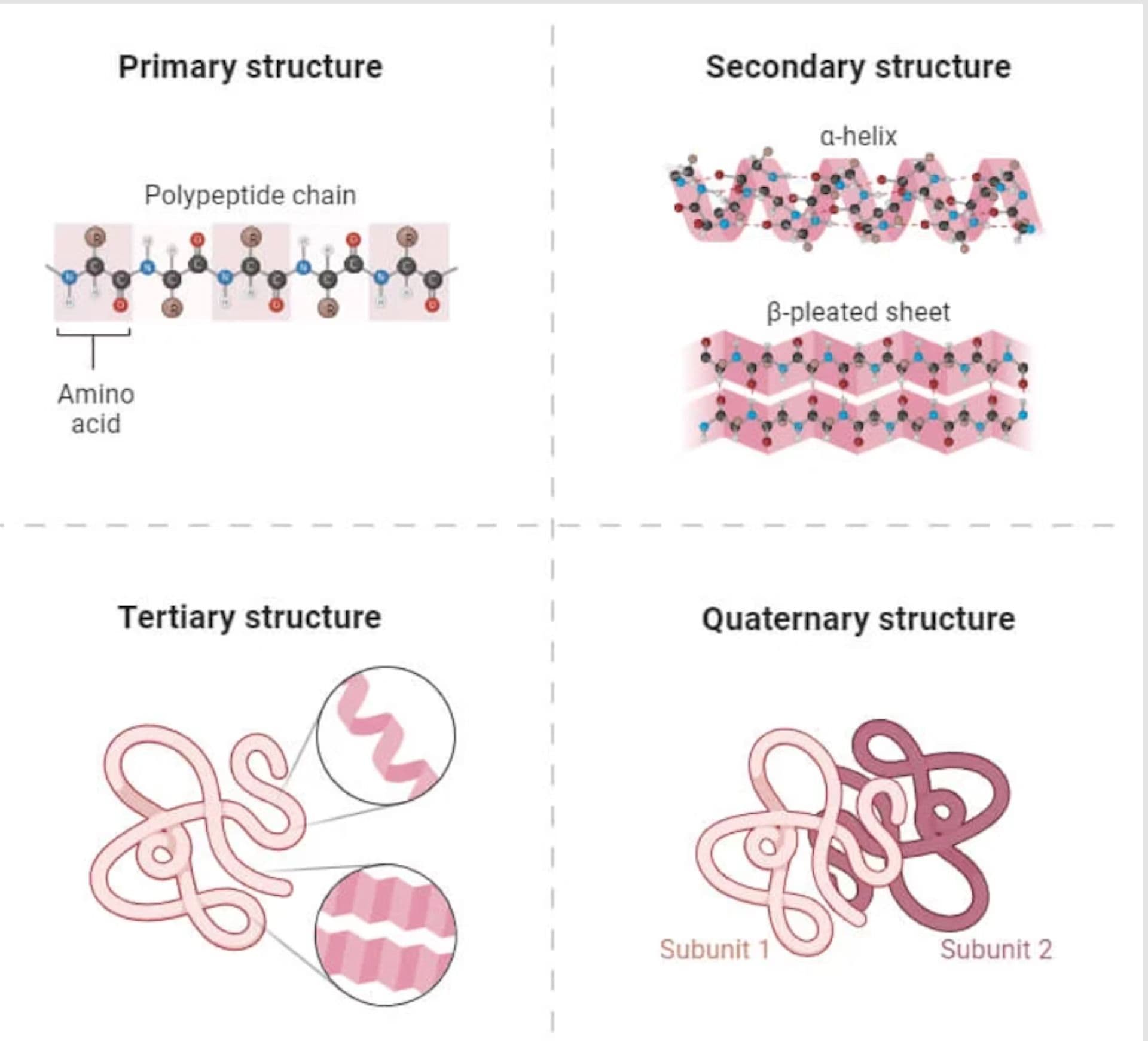
Proteins are composed of amino acid chains that twist and tangle into complex 3d structures guided by intermolecular interactions. While humans can figure out protein structures using experiments like X-ray crystallography and cryo-electron microscopy, these methods are time consuming, expensive and complex.
Predicting protein structures are important in nearly all fields of science from understanding diseases, improving drug design to biotechnology. Furthermore, knowing the 3D structure of proteins can not only improve understanding of how they interact with medicine, but also help in agriculture and environmental science.
AlphaFold’s success continued into 2021 where it again dominated at the CASP 14 and recognized as the solution to the grand challenge that spanned for more than 50 years.
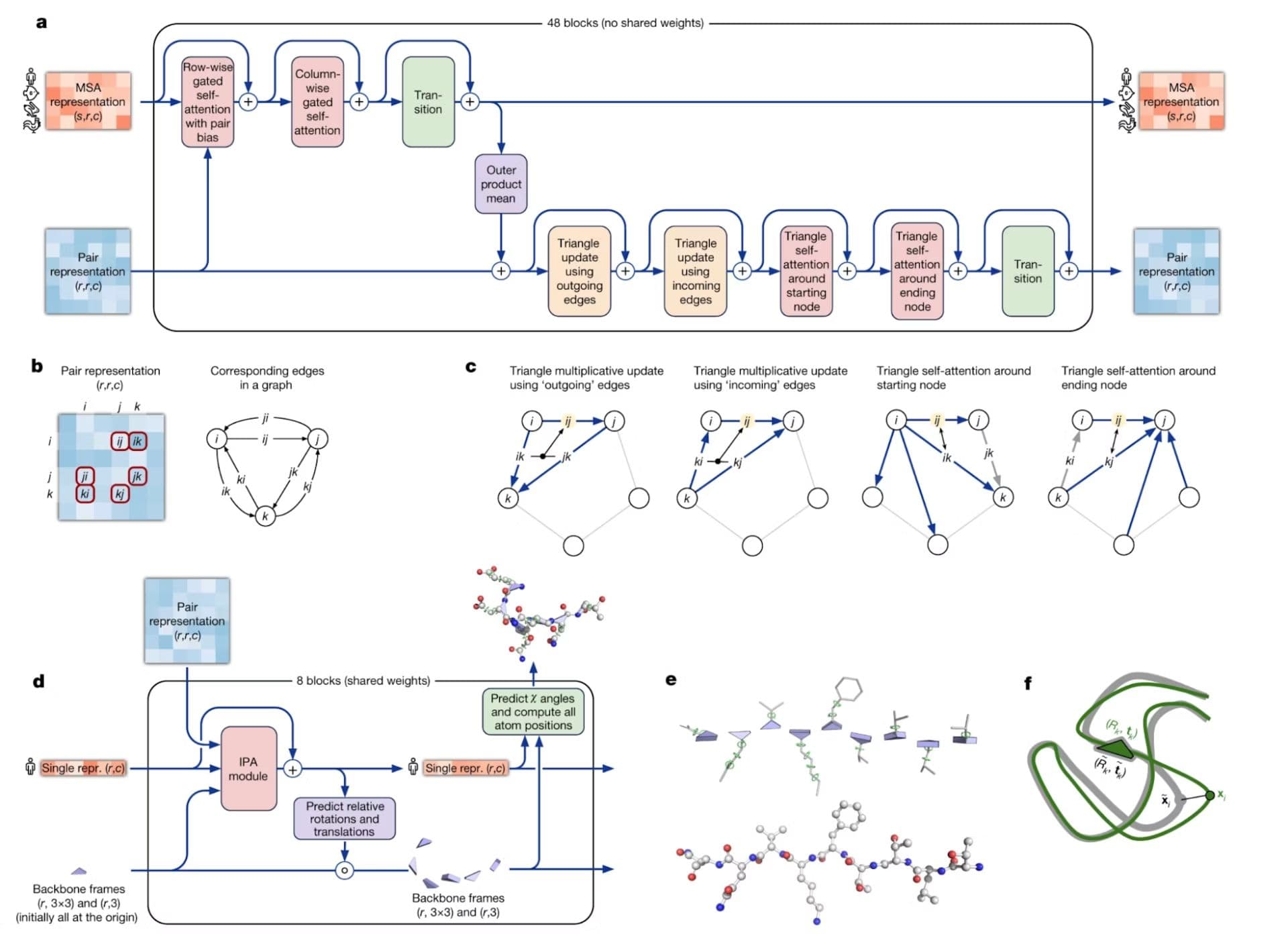
AlphaFold exploits the success of attention mechanisms in sequence modeling (yes, just like the self-attention used in Large Language Models!) to predict protein structures with high accuracy. The model treats the folded protein as a “spatial graph” with nodes and edges interacting in three dimensional space.
The prediction process begins by generating Multiple Sequence Alignments (MSAs) to provide evolutionary context, which helps the model understand crucial sequence regions. The neural network then creates a pair representation capturing amino acid relationships and predicts distances and angles between them.
This information then gets fed through blocks of “Evoformer”, a special Transformer block which utilizes self-attention to determine intricate relationships between amino acids.
Finally, the processed MSA and pair representations are taken through a “post-processing” module to complete the final three-dimensional model.
Today, AlphaFold has predicted more than 200 million protein structures—nearly all cataloged proteins known to science—and released the results in a public database, free for anyone to explore and use to assist in their research.
Med-PaLM
Med-PaLM is a groundbreaking large language model (LLM) developed by Google, specifically designed to tackle complex medical questions.
There is no doubt that with the increased ease of information access, there comes with risks and mistakes. This is especially apparent, and sometimes dangerous, with medical-related questions and diagnosis.
Google search has been known to provide outrageous diagnosis for the smallest problem, and with the frequent hallucination of Large Language Models such as ChatGPT, using LLMs as a personal doctor doesn’t seem appealing either.
The development of AI to answer medical-styled questions has been a challenging task for decades. Achieving success in this area could assist medical professionals in their jobs. Additionally, it could provide increased accessibility to reliable healthcare for those who cannot afford it.
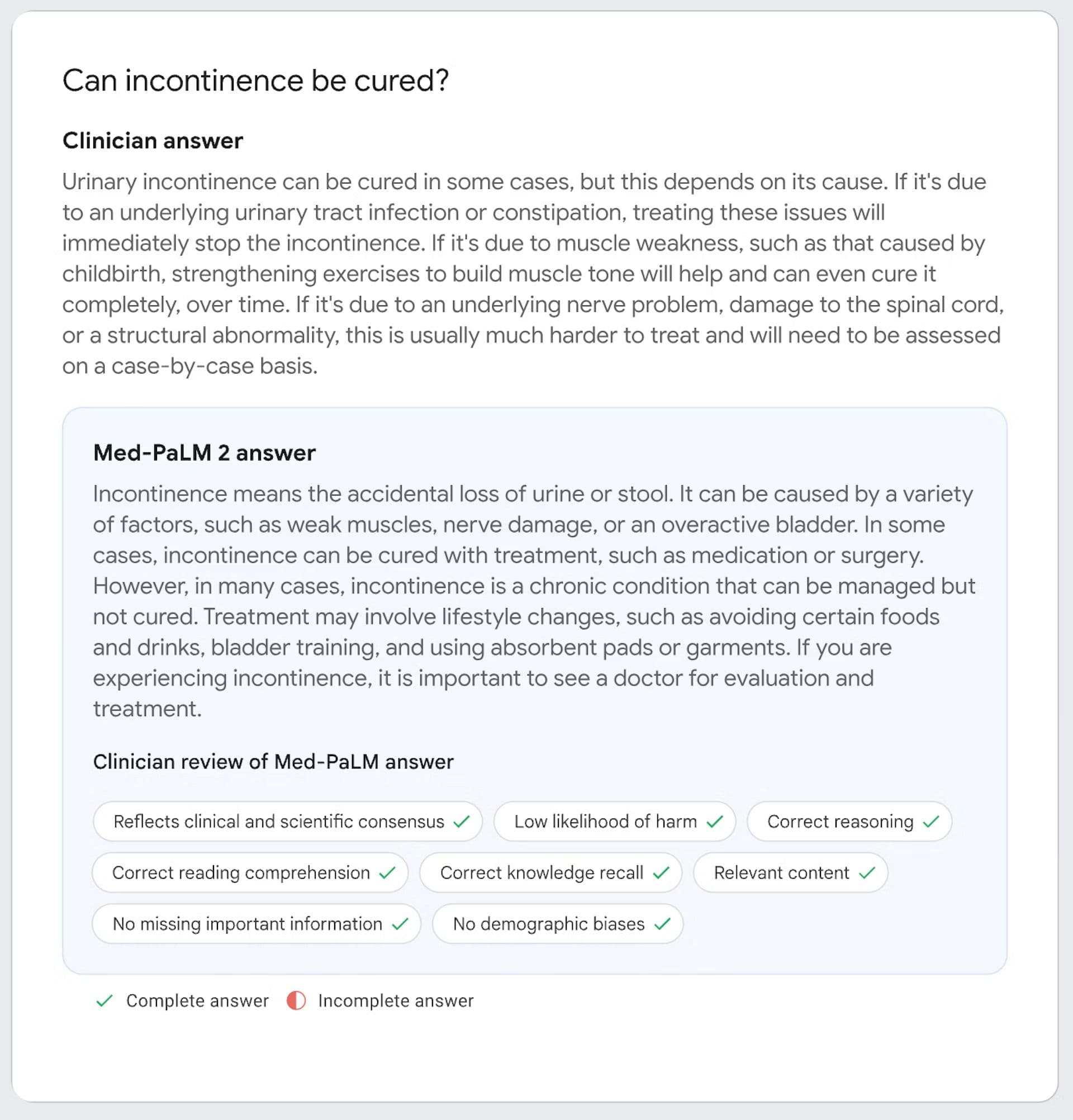
Google’s Med-PaLM series of models have gone beyond the traditional evaluation metrics in the field, which is mostly dominated by answering multiple-choice diagnosis questions, to accurately providing long-form responses.
These long form responses were not only evaluated on accuracy and adherence to instructions, but also the risk of harm if the answer was used in a real-life setting.
Med-PaLM’s answer evaluated by human experts
The Med-PaLM model was also extended with vision capabilities in the Med-PaLM M model, which can collect and synthesize insights from medical images such as X-Rays and mammograms.
The Med-PaLM models are based on the PaLM Large Language Model from Google. Instruction fine-tuning was applied with various Question Answer, or QA, datasets that are medical related. The resultant LLM is capable of answering both multiple choice and long response questions.
Unlike AlphaFold, medical LLMs such as MedPaLm is still in its early stages of development and although it performs well on artificial benchmarks, more work is still needed to ensure the safety and ethical concerns are eliminated.
Nova-2 Medical Model
Deepgram recently released the Nova-2 Medical Model, a speech-to-text model specifically designed to transcribe medical terms accurately into text.
Imagine Dr. Smith, a seasoned cardiologist, dictating patient notes after a long day of consultations. Without a specialized model, his speech recognition software transcribes “myocardial infarction” (a heart attack) as “my old car’s malfunction.” In another instance, “epidermolysis bullosa” (a rare skin condition) becomes “epic demolishes bull salsa.” The absurdity of these errors might be amusing in a casual context, but in the medical field, they could lead to dangerous misunderstandings and compromised patient care.
With speech-to-text being a technology that we have taken for granted for over a decade with personal assistants such as Siri or Google Assistant, people fail to realize how sensitive and fragile these technologies are under non-casual contexts.
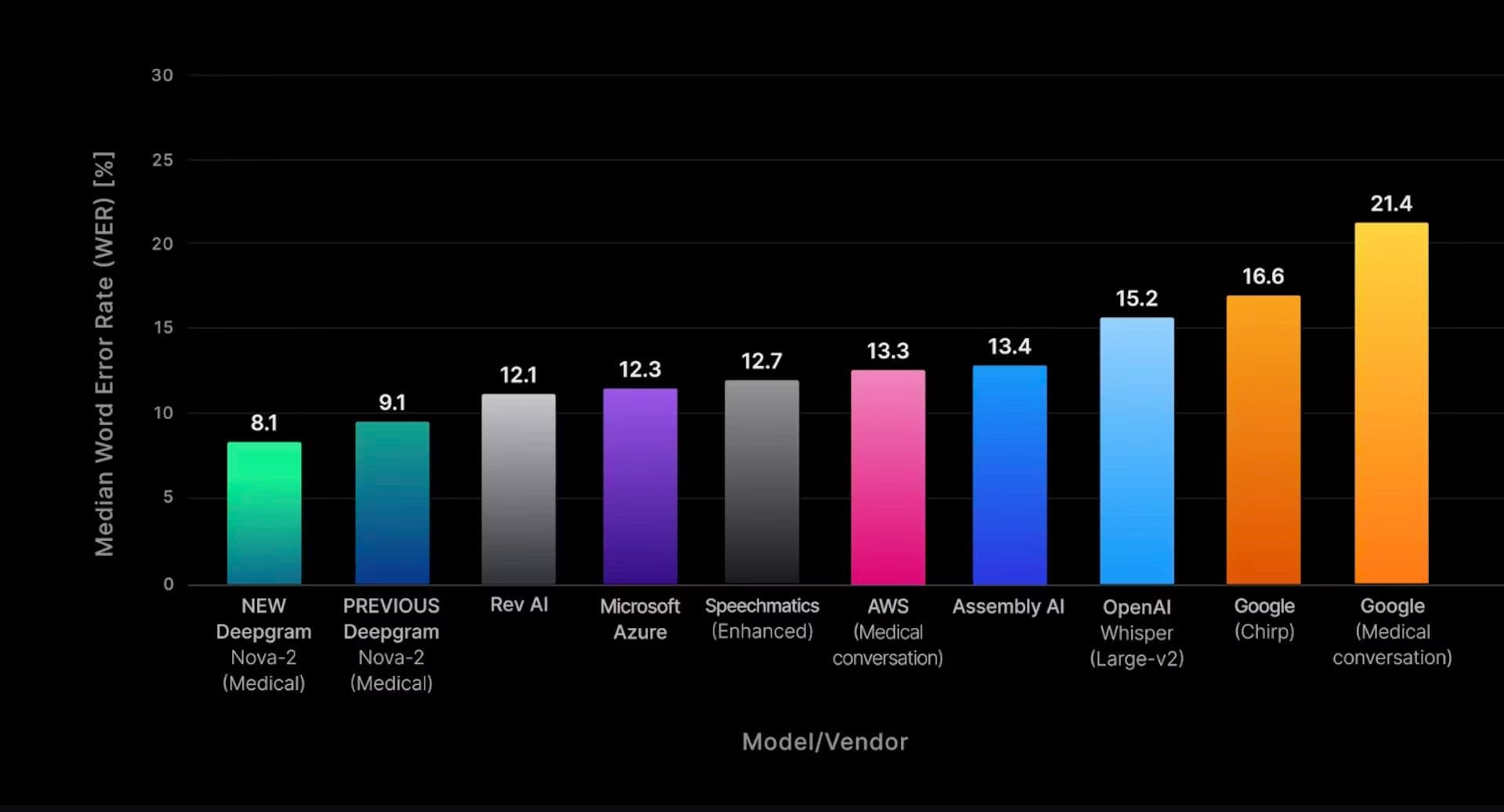
The Nova-2 Medical model represents a significant leap compared to other attempts at a specialized transcription model for medical jargons.
Median file word error rate (WER) for pre-recorded English transcription across all benchmarked medical domain test sets.
Similarly to Google’s Med-PaLM, specialized models like the Nova-2 Medical are based on a general-purpose baseline, such as the Nova-2 model. They are then trained on specific datasets, which creates a “spike” in knowledge for one specific area, giving rise to specialized models.
DiffDock
With Med-PaLM, we saw that LLMs can be a great helping hand in accessible patient care and preventing misuses in self-diagnosis made online. Surprisingly, the other category of “AI”, diffusion models, can play a critical role in the medical field as well.
Platforms such as DALL-E 2 and Midjourney utilizes diffusion models to generate stunning, realistic images based on the user’s inputs. However, researchers at MIT believe that the same mechanisms can be applied to accelerate the development of new drugs while reducing the risk of side effects.
DiffDock, the model that the researchers developed, aims to improve and automate the study of Molecular Docking. Molecular docking involves figuring out the interaction between a drug molecule and the protein that it aims to bind. Specifically, searching for an appropriate “pose” to allow the drug molecule to bind to the target.
The diffusion process of DiffDock
Traditionally, this was mostly done through brute force testing and experimentation. While it has led to the development of novel drugs, the cost can be up to billions for a single drug and the failure rate is high.
By utilizing generative methods like diffusion models, instead of assuming a single “correct” pose to be predicted for one drug molecule, generative methods can generate multiple configurations with different probabilities of success.
DiffDock was able to identify multiple binding sites on the target protein that it has never encountered before. It also performs significantly better than previous methods, predicting an accurate pose 22 percent of time in testing compared to the mere 10 percent for other docking models.
Exscientia
Rather than a single machine learning model, Exscientia is a British startup focused on utilizing AI for drug design. Their promising research sparked success in 2020 when a clinical trial was approved for the first ever AI designed drug.
The development time was sped up by nearly 5 fold using AI, with the 2020 clinical trial taking place a mere 12 months after development compared to the average time of 5 years.
The drug was designed to be a faster and more effective way to treat obsessive-compulsive disorder (OCD), with the molecule identified by a machine learning model.
Not long after the first clinical trial in 2020, a second molecule was identified to treat Alzheimer’s disease entered phase 1 of clinical trial. Exscientia’s framework is agnostic, meaning that it can be applied to any disease, not just a specific category.
Exscientia has obtained success with their clinical trials and it’s not just empty promises.
The latest achievement is from the EXALT-1 trial, a groundbreaking study demonstrating the effectiveness of Exscientia’s AI-supported precision medicine platform for treating advanced hematological cancers.
The study, published in Cancer Discovery, showed that 54% of patients experienced a clinical benefit, with progression-free survival (PFS) extended by more than 1.3-fold compared to their previous therapies. Even more impressive, 40% of patients had exceptional responses, lasting at least three times longer than expected for their respective diseases.
This trial utilized Exscientia’s single-cell functional precision medicine (scFPM) platform, which tests drug responses ex vivo using patients’ own tissue samples. By accurately predicting the most effective therapy for each individual, this approach exemplifies the potential of AI in personalizing cancer treatment.
Pharma.AI
Insilico Medicine’s Pharma.AI leverages AI to streamline the drug discovery process. They have multiple models that optimize different parts of the entire drug discovery process.
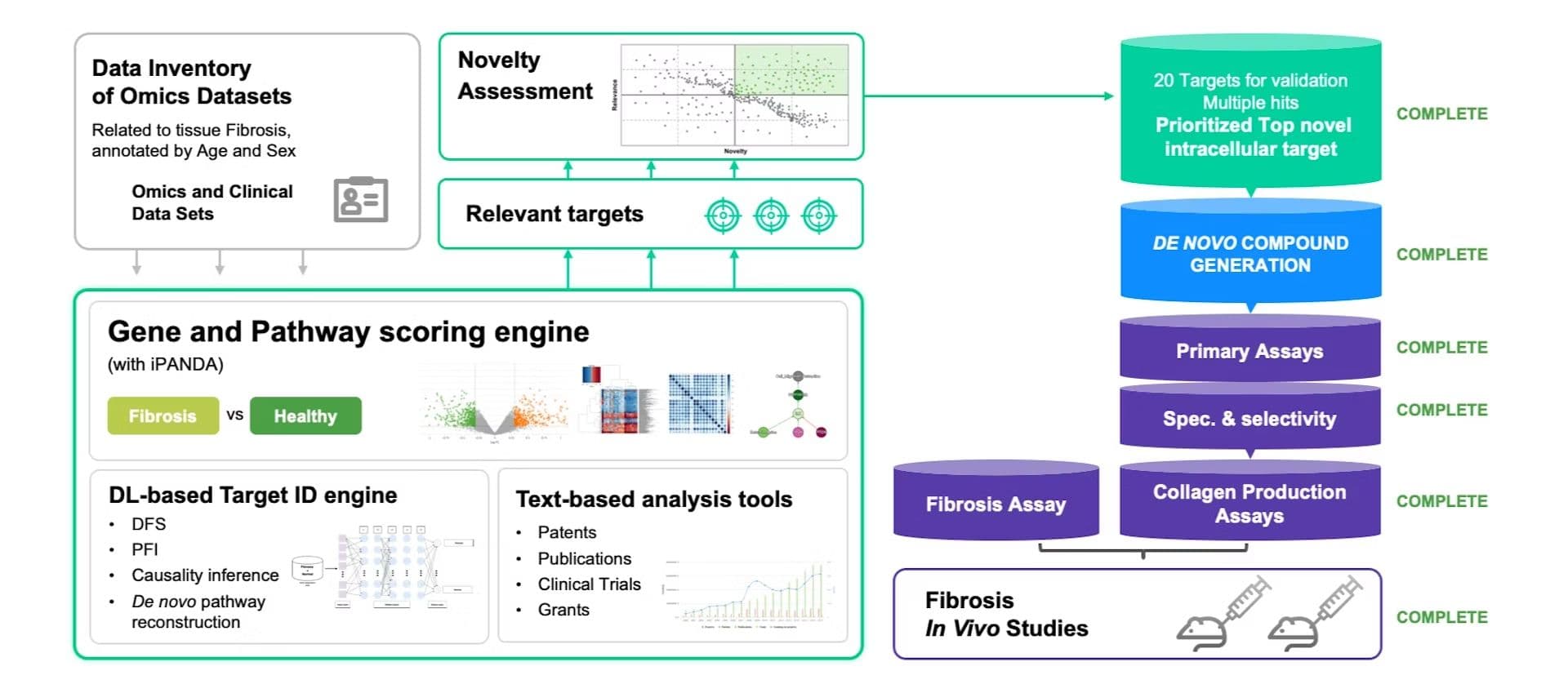
Their platform PandaOmics integrates multi-omics data to identify disease signatures and prioritize drug targets, successfully uncovering novel targets for conditions like fibrosis and cancer.
Once targets are identified, Chemistry42 uses AI for de-novo drug design, generating novel molecules with optimized properties.
Once novel drugs are identified, inClinico enhances clinical trial success rates by predicting outcomes and optimizing trial designs, improving patient selection and overall efficiency.
In 2022, Pharma.AI's suite of models achieved a major milestone with the development of the drug candidate ISM001-055 for Idiopathic Pulmonary Fibrosis (IPF), a severe lung disease.
Using PandaOmics, Insilico Medicine analyzed omics and clinical data related to fibrosis, identifying 20 potential targets. From these, a novel intracellular target was prioritized. Chemistry42 then designed a series of molecules to bind to this target, resulting in ISM001-055, which demonstrated strong efficacy in preclinical studies.
Pharma.AI's pipeline for drug discovery
After successful Phase 0 trials in healthy volunteers, ISM001-055 moved to Phase 1 clinical trials, showing promising pharmacokinetic and safety profiles, a significant achievement in AI-driven drug development.
The entire process was not only successful in preclinical studies, but the cost was around $2.6 million, orders of magnitude less than the billions needed with traditional drug discovery techniques.
Conclusion
Of course, an article is far cry from being enough to cover and justify the work done by researchers in applying Artificial Intelligence to the medical field. In just the drug discovery area alone, there have been many other companies and research firms taking success. For example, Absci was able to create and validate De Novo Antibodies with Zero-Shot Generative AI, accelerating the time to clinic by 50%.
On the other hand, medical image analysis is being pioneered by machine learning as well. From cancer screening to detecting neurological disorders, there are endless possibilities.
The future of AI in the medical field is incredibly promising. As AI evolves, its integration into medical research and practice will lead to groundbreaking discoveries and innovations. By enhancing diagnostics, personalizing treatments, and accelerating drug development, AI can significantly improve patient outcomes and make healthcare more accessible.
While generative AI captures public imagination, the steady advancements in medical AI are transforming lives. Collaboration between AI researchers and medical professionals will be crucial to unlocking these technologies’ full potential, addressing pressing health challenges ethically and effectively.